Solutions are formed when a solute is dissolved in a solvent.
Forming solutions
In order for a solution to form, the solute intermolecular forces must be broken as well as the solvent intermolecular forces. Then the solute and solvent form new intermolecular forces with each other. If the energy required to break the intermolecular forces is much greater than the energy released when the new forces are formed, the solution will not form.
Factors affecting solubility
For gases, as the pressure of the gas above the solution increases, the solubility of the gas increases. For gases, as the temperature of the solution increases, the solubility of the gas decreases. For most solids, as temperature increases, the solubility increases.
Concentration calculations
There are many ways to express concentration (which is the ratio of solute to solvent or solution).
% by mass: 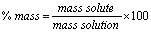
The mass units must match!
% by volume: 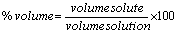
The volume units must match!
% mass/volume: 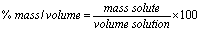
The volume unit is mL
Molarity (M): 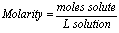
Molality (m): 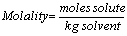
A sample becomes diluted (less concentrated) when more solvent is added. The dilution equation is
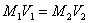 M1 = original molarity V1 = original volume M2 = new molarity V2 = new volume. Volume units must match!
M1 = original molarity V1 = original volume M2 = new molarity V2 = new volume. Volume units must match!
Colloids
Colloids are solutions with solute particles large enough to scatter light. They exhibit the Tyndall Effect, where light is seen traveling through and spreading out as it travels through colloid.



