Energy & Physical Changes
| Topic Review on "Title": |
Physical and chemical changes occur with changes in heat.
Energy, heat and temperature
Several definitions are useful in understanding thermodynamics:
- Energy: The ability to do work or supply heat.
- System: Particles under-going change.
- Surroundings: Everything surrounding the system.
- Temperature: proportional to the average kinetic energy of the particles.
- Heat (q): Flow of energy from a hotter object to a cooler object.
- Enthalpy (H): Takes into account internal energy, pressure and volume. In an open-air environment, it’s the same as heat.
Physical changes
The specific heat capacity is the amount of energy that can be absorbed before temperature begins to change. 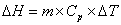 where m = mass; Cp = specific heat capacity and DT = T2 – T1. During changes in state, the heat of fusion and heat of vaporization are used. Melting: where m = mass; Cp = specific heat capacity and DT = T2 – T1. During changes in state, the heat of fusion and heat of vaporization are used. Melting: 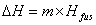 Hfus = enthalpy of fusion. Boiling: Hfus = enthalpy of fusion. Boiling: 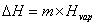 Hvap = enthalpy of vaporization. Heating curves show the combination of the processes—changing temperatures and changing states. Hvap = enthalpy of vaporization. Heating curves show the combination of the processes—changing temperatures and changing states.
Calorimetry
When the system loses energy, the surroundings gain it and vice versa. Therefore, the energy change in the surrounding can be measured and used to determine information about the system.
|
| Rapid Study Kit for "Title": |
| Flash Movie |
Flash Game |
Flash Card |
| Core Concept Tutorial |
Problem Solving Drill |
Review Cheat Sheet |
 |
 |
 |
|
| "Title" Tutorial Summary : |
Thermodynamics is the study of heat changes during processes. The heat changes of physical processes will be introduced using specific heat capacity, heats of fusion and vaporization, and calorimetry.
|
| Tutorial Features: |
Series Features:
- Concept map showing inter-connections of new concepts in this tutorial and those previously introduced.
- Definition slides introduce terms as they are needed.
- Visual representation of concepts
- Animated examples—worked out step by step
- A concise summary is given at the conclusion of the tutorial.
|
| "Title" Topic List: |
- Energy, temperature and heat
- Energy and physical changes
- Specific heat capacity
- Calorimetry
- Heat of fusion
- Heat of vaporization
- Heating curves
|
See all 24 lessons in college chemistry, including concept tutorials, problem drills and cheat sheets:
Teach Yourself HighSchool Chemistry Visually in 24 Hours |



