Acids and bases are used throughout chemistry.
Definitions of acids and bases
There are three common definitions of acids and bases:
- Arrhenius acid: Produces hydronium ion in water.
- Arrhenius base: Produces hydroxide ion in water.
- Brønsted-Lowry acid: Donates a proton (H+1)
- Brønsted-Lowry base: Accepts a proton
- Lewis acid: Accepts electrons
- Lewis base: Donates electrons
Strong acids and bases are ones in which most molecules perform their “duty” while weak acids and bases only have a few acid and base molecules that act as acids and bases. There are only a few strong acids and bases to remember—the rest will most likely be weak. Strong acids: HCl, HBr, HI, HNO3, HClO3, HClO4. Strong bases: NaOH, KOH, Ca(OH)2, Ba(OH)2, Sr(OH)2
A conjugate acid is what remains after a base does its “job”. A conjugate base is what’s left after an acid does it’s “job.” Strong acids or bases form a weak conjugate and vice versa.
Equilibrium of acids and bases
Equilibrium constants can be written for acid and base dissociation reactions. Water also autoionizes to form hydrogen and hydroxide ions. The equilibrium constant for the acid dissociation reaction ´ the base dissociation constant for the conjugate base = the water dissociation constant at that temperature.
pH
The pH scale is a logarithmic scale to measure the acidity of a solution. 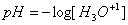 Strong acids and bases can be assumed to dissociate completely. Therefore, the concentration of the original strong acid or base is assumed to equal the concentration of the hydrogen or hydroxide ion. For weak acids or bases, the equilibrium constant and ICE charts are used to determine the concentration of the hydronium ion before solving for pH.
Strong acids and bases can be assumed to dissociate completely. Therefore, the concentration of the original strong acid or base is assumed to equal the concentration of the hydrogen or hydroxide ion. For weak acids or bases, the equilibrium constant and ICE charts are used to determine the concentration of the hydronium ion before solving for pH.
Acid/base properties of salts
Some salts can have acid/base properties based on the acid or base they are based off of.
Salts from
- Weak acid + strong base = Basic
- Weak acid + weak base = Neutral
- Strong acid + weak base = Acidic
- Strong acid + Strong base = Neutral
Buffers
A buffer is a solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers resist changes in pH when acids or bases are added. The pH will still change, but much less than if it was plain water. Buffers use the Henderson-Hasselbach equation: 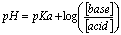
Titrations
Titrations are used to find the concentration of an unknown solution using a solution of known concentration. An indicator is used that changes color at the stoichiometric point (the point at which no reactants are left over) based on the pH of the products that are in solution at that point. Stoichiometry is used at that point to determine the unknown concentration.



